TRANSFERENCE OF PROTEINS ON A MEMBRANE AND SPECIFIC DETECTION WITH ANTIBODIES
The process can be divided into 3 steps:
The proteins, separated by a SDS-PAGE, are transfered onto a membrane applying an electric field.
|
The blue cooling unit has to be froozen @ -20 °C The Transfer-buffer has to be stored at 4 °C Use gloves, do not touch the membrane |
 |
|
1) Cut the filter paper and blotting membrane to the size of the gel (10 x 7.5 cm) 2) Soak NC-membrane, filter paper and fiber pad for 15 min in ice-cold Transfer-buffer Equilibrate the gel for 15 min (not longer!) in ice-cold Transfer-buffer PVDF-membranes have to be activated with MeOH for 2(15?) min previously 3) Place cassette with grey side down into a box Place soaked fiber pad on Cassette Place soaked filter paper on fiber pad use 1 filter paper from Biorad or 3 thin filter papers Place equilibrated gel on filter paper Place soaked membrane on gel Use glass tube to remove air bubbles Place soaked filter paper on membrane Place soaked fibre pad on filter paper Close clear tap and fix the gel sandwich Do not move sandwich during locking the Cassette |
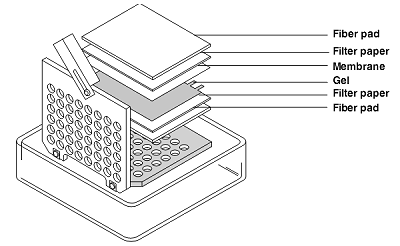 |
|
4) Place the Cassette into the module: grey side of the Cassette to the black part of the Module (do the same for the second cassette, if necessary) 5) Place the Module into the Tank Beware the orientation: Black side of the Module to the black mark from the Tank 6) Place the frozen blue cooling unit in front of the Module into the Tank 7) Fill into the Tank the Transfer-buffer up to the "Blotting"-mark (~ 450 ml?) 8) Place a magnetic stirrer into the Tank. Keep buffer stirred during blotting. 9) Connect system to Power supply (beware of the orientation / colour code) 10) Start blotting: 12 hours: 30 V, constant 100 mA (36 Wh) 3 hours: 60 V, constant 200 mA (36 Wh) 1 hour: 100 V, constant 350 mA (35 Wh) use constant current: if the ionic strength gets too high, the voltage drops, thus preventing overheating; only the transfer time increases much. (images are taken from the instruction manual) |
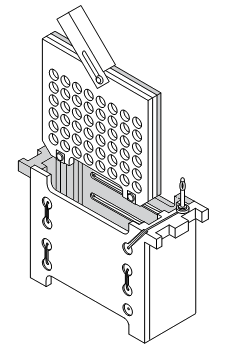 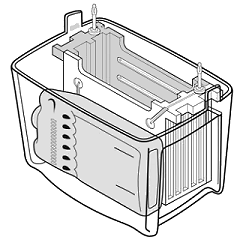 |
| Buffer | Comments | |
| TBST | cheap, w/o biotin; some background, may release prot. from membr. | |
| TBST, 1% Tween or Triton X-100 |
cheap, w/o biotin; some background, may release prot. from membr. | |
| Blotto 5% milk powder in TBST |
cheap, w/o background; w/ biotin, may release prot. from membr. | |
| 1-3% BSA Cohn V grade in TBST |
w/o background; expensive, w/ biotin |
|
13) Prepare 50 ml(?) Blotto for each membrane. 14) Transfer 30 ml Blotto into a clean tray. 15) Place membrane with gel-faced side upwards(?) into Blotto. 16) Incubate 1 h @ RT, shaken (gyro-shaker) |
 |

| Chromogene | Comments | |
| 4-chloronaphtol (4-CN) + H2O2 |
cheap, blue/black bands; intensity diminish fast | |
| 3,3'-diaminobenzidine (DAB) + CoCl2 + H2O2 |
cheap, black bands; high background | |
| Chemiluminescence ECL or (ECL+) |
(+++++) |
very sensitive, good documentation; expensive, requires system for develop fotos |
| Chromogene | Comments | |
| 5-bromo-4-chloro-3-indolyl phosphate (BCIP) + Nitro blue tetrazolium (NBT) |
cheap, good documentation; requires DMF as solvent, intensity diminish slowly |
|
17) Wash 3 x 10 min @ RT with 50 ml TBST. 18) Primary antibody: dilute in ~20 ml (8 ml in a bag) TBST (or Blotto) and incubate 1-6 h @ RT, shaken (gyro-shaker). 19) Wash 3 x 10 min @ RT with 50 ml TBST. 20) Secondary antibody: dilute in ~20 ml (8 ml in a bag) TBST (or Blotto) and incubate 1-2 h @ RT, shaken (gyro-shaker). 21) Wash 3 x 10 min @ RT with 50 ml TBST. 22) Add activated DAB solution to membrane and incubate @ RT until desired development is archieved (typically 5-15 min) |
 |