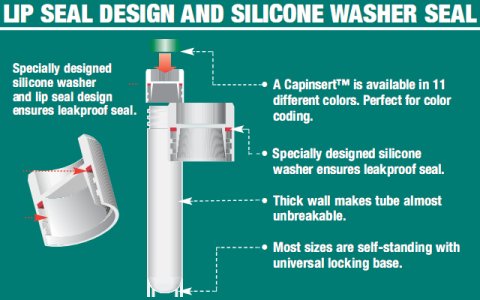
To achieve that the cells do not suffer from the congelation process, either by ice crystall formation or by dehydration, the cells have to be:
a) suspendend in freezing medium (typically 85% medium, 10% FBS, 5% DMSO)
b) cooled down slowly (1 °C/min) to -80 °C before transfered to the LN2
A low tech method to achieve this cooling rate is "Mr. Frosty" from Nalgene (#5100): This is a polycarbonate box with a PE vial holder for 18 cryovials which has to be filled up with 200 ml isopropanol. Precooled (4 °C), the cryovials containing the cells are placed into it and stored overnight at -80 °C and then transfered fastly (to avoid decongelation) into the LN2.
As we do not have a -80 °C ultracongelator, we have to freeze the cells at -20 °C before transfering into the LN2. At least with the "easy" to handle HeLa cells this works.
|
 |