Solutions:
Acetate-buffer: 1.59 g NaAc + 16 ml HAc in 1 l A. dest. (300 mM, pH 3.6)
HCl: 83 ml A. dest. + 500 痞 25% HCl (40 mM)
TPTZ (2,4,6-Tris(2-Pyridyl-s-TriaZine): 31.2 mg TPTZ in 10 ml 40 mM HCl (10 mM)
FeCl3: 54 mg FeCl3 * 6 H2O in 10 ml A. dest. (20 mM)
FRAP: 25 ml acetate-buffer, 2.5 ml TPTZ (0.83 mM), 2.5 ml FeCl3 (1.67 mM)
Procedure:
- 100 痞 sample, pos. (Trolox) or Mock (acetate-buffer) control
(unknown sample: 1, 10 & 100 痞 and dilute w/ buffer)
- 900 痞 FRAP
- mix, incubate 2 h at 37 蚓, dark? (0.75 mM TPTZ, 1.5 mM FeCl3)
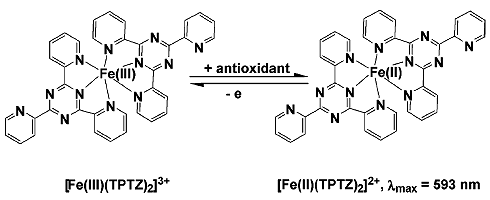
- measure absorbance @ 593 nm (formed FeII(TPTZ)2)
- Standard: 0-50-100-200-300-400-500 然 Trolox
Antioxidant Power = ASample - AMock - ANeg.
with different sample concentrations EC50 can be calculated (Dose-Response curve).
Troubleshooting:
- Preparation of FeCl3-soln
|
original FRAP paper
|